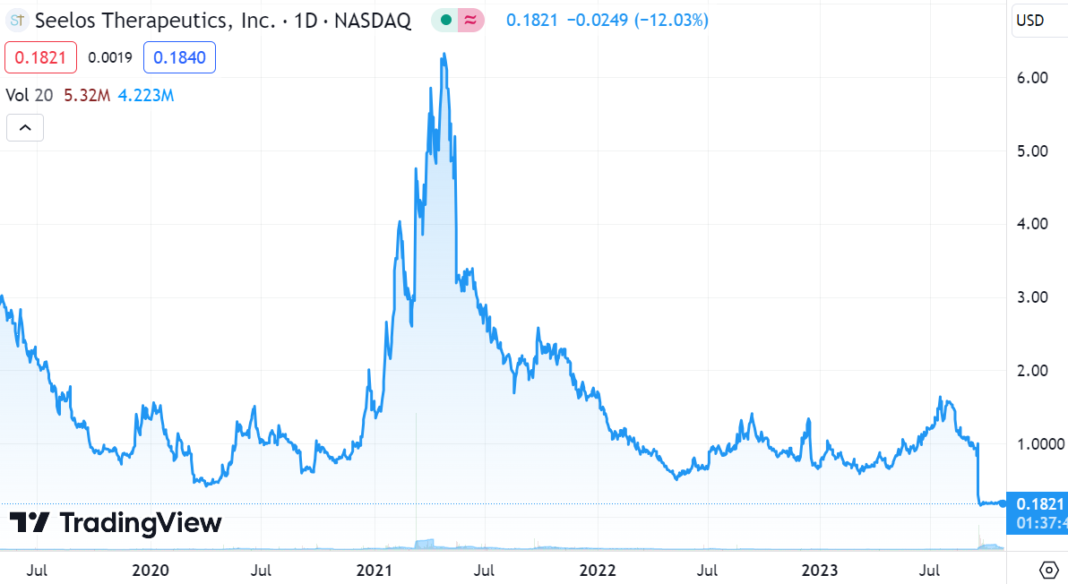
It’s a jungle out there. Trending sideways for second week.
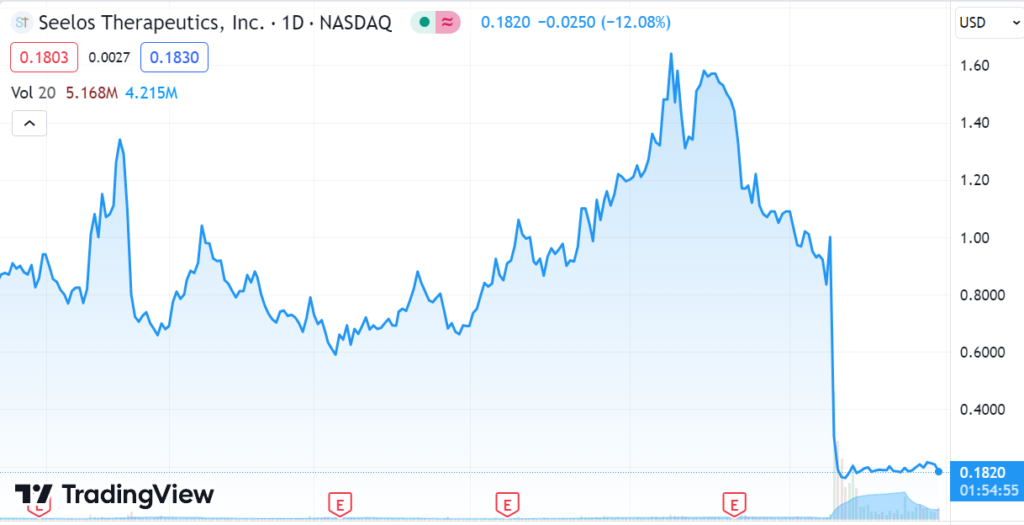
Company version of trial (September 20th): Seelos Therapeutics Announces Top Line Results
Company says, “..the study would have achieved statistical significance for the primary endpoint, had the study reached full enrollment (220 patients) versus 147 patients. Well alrighty then..
Endpoint version of trial: Seelos’ Stock Plunges by Over 75% After Ketamine Drug for Suicide Prevention Fails a Phase II Trial
After the Dust Settles: Seelos Therapeutics Retains Canaccord Genuity.
The reason for the earlier optimism is if approved, SLS-002 would become only the second product approved specifically for ASIB (acute suicidal ideation and behavior), after Johnson & Johnson’s rapid-acting Spravato (esketamine) was approved for moderate-to-severe MDD patients with ASIB in 2020 in the US.
Spravato’s high cost of therapy and requirement for two hours of patient observation after administration will limit its uptake. As such, GlobalData forecasts that Spravato will generate global sales of approximately $383 million by 2029.
As a related compound, and with the same intranasal route of administration, it is possible that SLS-002 will face similar issues to Spravato. However, in a small randomized trial, intranasal ketamine caused minimal dissociative effects, which, if replicated in larger trials, would give SLS-002 an advantage over Spravato.
The CDC estimates there were more than 49,000 suicides in the US in 2022, representing the highest number recorded in the country’s history. There are no FDA-approved treatments for suicidality in major depression
Forward Looking Statements
Statements made in this post, which are not historical in nature, constitute forward-looking statements for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. These statements include, among others, those regarding the safety and efficacy of SLS-005 and its ability to clear only mutant, toxic, and aggregating proteins in the body and its potential as a pipeline-in-a-product. These statements are based on Seelos’ current expectations and beliefs and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Risks associated with Seelos’ business include, but are not limited to, the risk of not successfully executing its preclinical and clinical studies and not gaining marketing approvals for its product candidates, the risk that prior clinical results may not be replicated in future studies and trials (including the risk that the results from the preclinical in-vivo studies of SLS-005 or non-human studies are not replicated or are materially different from the results of future studies and trials), the risks that clinical study results may not meet any or all endpoints of a clinical study and that any data generated from such studies may not support a regulatory submission or approval, the risks associated with the implementation of Seelos’ business strategy, the risks related to raising capital to fund its development plans and ongoing operations, risks related to Seelos’ current stock price and listing on the Nasdaq Capital Market, as well as other factors expressed in Seelos’ periodic filings with the U.S. Securities and Exchange Commission, including its most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we do not know whether our expectations will prove correct. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, even if subsequently made available by us on our website or otherwise. We do not undertake any obligation to update, amend or clarify these forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Contact Information:
Anthony Marciano
Chief Communications Officer
Seelos Therapeutics, Inc. (Nasdaq: SEEL)
300 Park Avenue, 2 nd Floor
New York, NY 10022
(646) 293-2136
[email protected]
https://seelostherapeutics.com/
https://twitter.com/seelostx
https://www.linkedin.com/company/seelos
Mike Moyer Managing Director
LifeSci Advisors, LLC
250 West 55th St., Suite 3401
New York, NY 10019
(617) 308-4306
[email protected]