Conference Call Today at 12:00 EST.
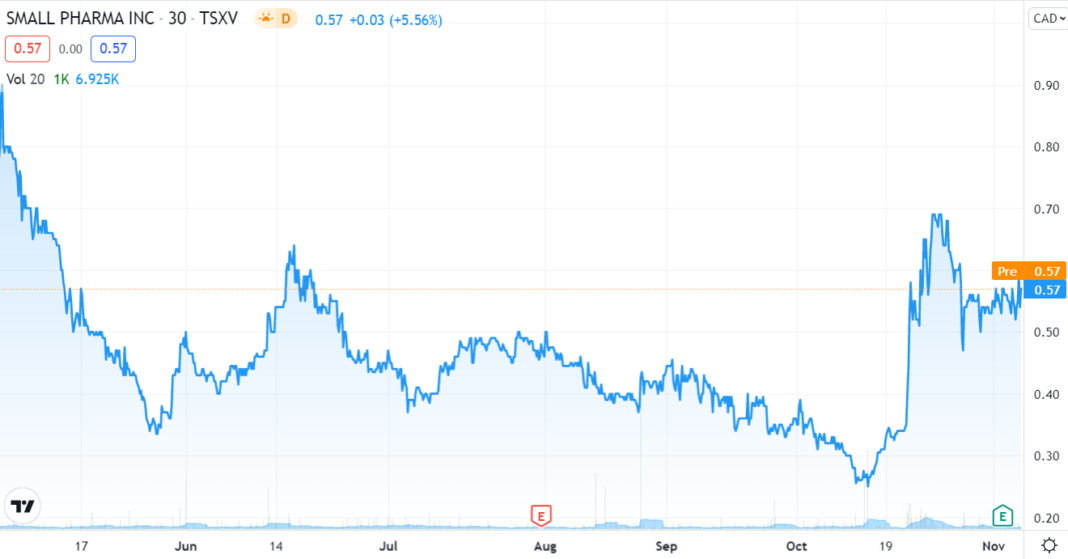
As the TSX chart above show, investors appear to be warming up the the idea.
Not many investors have heard of London based Small Pharma in the Psychedelic sector which itself is relatively unknown, unlike the Biotech sector. One could say this makes the company ‘doubly’ unknown, a good thing for long-term investors – assuming their considerable progress continues.
Please join LifeSci Advisors for “Lunch With LifeSci”, November 4th at 12:00 PM ET featuring Small Pharma (DMTTF). To register and attend click here.
Conducting the world’s first DMT clinical trial for Major Depressive Disorder:
With a market cap of $154M, $43M cash and 317M shares outstanding, Small Pharma and their clinical candidate, SPL-026, is one of the shortest acting psychedelics being investigated under regulatory clinical trials today.
Small Pharma’s DMT formulation induces a temporary and short (circa 15-30 minutes) altered state of consciousness from the safe confines of a health clinic. This has been linked to brain rewiring that may provide an opportunity to break unhealthy patterns of negative thinking common in depression, allowing for new positive thoughts to form, with the support of talk therapy.
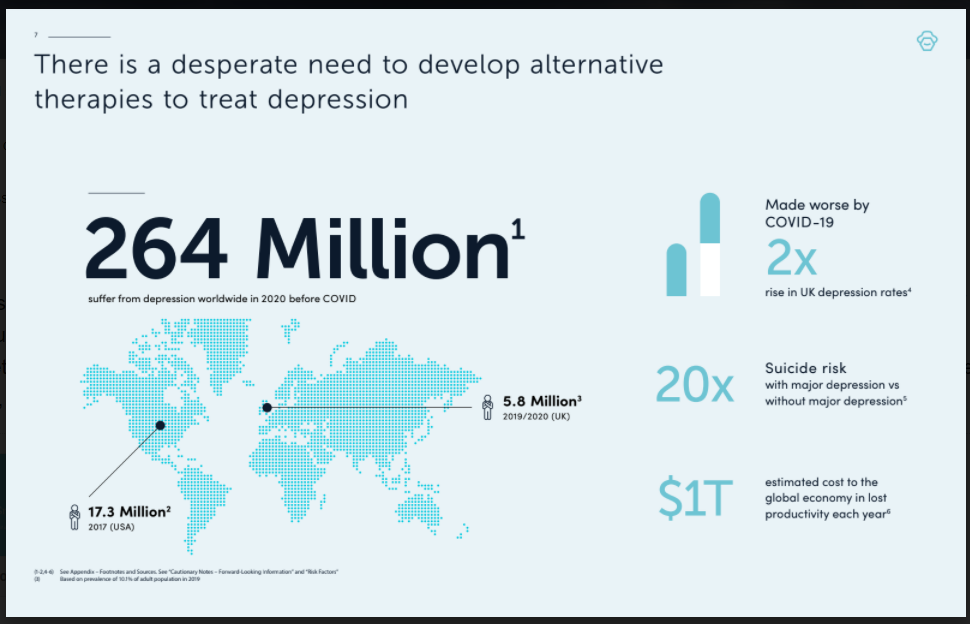
Given the mental health crisis compounded by COVID-19, millions of patients are in desperate need of help. While current antidepressants deliver only symptom suppression, Small Pharma’s DMT trial is investigating whether this treatment, by targeting the root causes of depression, can rapidly deliver long lasting relief.
Website: Small Pharma
Everything You Need to Know About DMT, the ‘Spirit Molecule.’ Healthline
Over 14,000 Psychedelic Investors have downloaded the 2021 Psychedelic Investor Guide.
Subscribe to get your copy.
For further information contact:
Small Pharma Inc.
Peter Rands
Chief Executive Officer
Email: [email protected]
Tel: +44 (0)2071 129118
Media Relations Contact
McKenna Miller
KCSA Strategic Communications
Email: [email protected]
Tel: +1 (949) 949-6585
Investor Relations Contacts
Eric Ribner
LifeSci Advisors, LLC
Email: [email protected]
PRESENTATION DISCLAIMER
Cautionary Note Regarding Forward-Looking Statements
This press release contains statements that constitute “forward-looking information” (“forward-looking information”) within the meaning of the applicable Canadian securities legislation. All statements, other than statements of historical fact, are forward-looking information and are based on expectations, estimates and projections as at the date of this news release. Any statement that discusses predictions, expectations, beliefs, plans, projections, objectives, assumptions, future events or performance (often but not always using phrases such as “expects”, or “does not expect”, “is expected”, “anticipates” or “does not anticipate”, “plans”, “budget”, “scheduled”, “forecasts”, “estimates”, “believes” or “intends” or variations of such words and phrases or stating that certain actions, events or results “may” or “could”, “would”, “might” or “will” be taken to occur or be achieved) are not statements of historical fact and may be forward-looking information. Forward-looking statements in this news release include statements regarding the Company’s placebo-controlled, proof-of-concept Phase IIa study of SPL026, the anticipated timing for the readout of topline data for the Company’s Phase IIa trial, the anticipated commencement and timing of the Company’s Phase IIb trial of SPL026, the completion of the Company’s pre-clinical studies and trials and assessment of the efficacy of the trials using the Montgomery-Asberg Depression Rating scale, the Company’s success in launching a clinical program into DMT-assisted therapy, the Company’s ability to develop solutions to effectively address depression through DMT-based therapies, the potential of DMT-assisted therapies to transform the lives of patients suffering with MDD, the acceleration of SPL026’s time to market and facilitation of patient access to medicines for emerging and novel treatments, the Company’s access to a toolkit to support all stages of the design, development and approvals process, as well as to identify key areas for future engagement under the ILAP, enhanced coordination and monitoring of important product development activities culminating in market authorization as a result of the ILAP, the Innovation Passport Designation providing potential access to speedier and more efficient development pathways for SPL026, current and future status of the Company’s patent application, rapid advancement and build-out of the Company’s portfolio, the Company’s ability to innovate and develop novel treatment candidates for mental health conditions through ketamine-based candidates, and the Company’s ability to accelerate the development of novel treatment to mental health conditions. Although the Company believes that the expectations reflected in such forward-looking information are reasonable, it can give no assurance that the expectations of any forward-looking information will prove to be correct. Known and unknown risks, uncertainties, and other factors which may cause the actual results and future events to differ materially from those expressed or implied by such forward-looking information. Such factors include, but are not limited to: compliance with extensive government regulations; domestic and foreign laws and regulations adversely affecting the Company’s business and results of operations; the impact of COVID-19; and general business, economic, competitive, political and social uncertainties. Accordingly, readers should not place undue reliance on the forward-looking information contained in this press release. Except as required by law, the Company disclaims any intention and assumes no obligation to update or revise any forward-looking information to reflect actual results, whether as a result of new information, future events, changes in assumptions, changes in factors affecting such forward-looking information or otherwise.
Small Pharma makes no medical, treatment or health benefit claims about its proposed products. The MHRA or other similar regulatory authorities have not evaluated claims regarding DMT-assisted therapies and other next generation psychoactive compounds. The efficacy of such therapies has not been confirmed by MHRA-approved research. There is no assurance that such DMT-assisted therapies and other psychoactive compounds can diagnose, treat, cure or prevent any disease or condition. Vigorous scientific research and clinical trials are needed. Any references to quality, consistency, efficacy and safety of potential therapies do not imply that Small Pharma verified such in clinical trials or that Small Pharma will complete such trials. If Small Pharma cannot obtain the approvals or research necessary to commercialize its business, it may have a material adverse effect on Small Pharma’s performance and operations.
This news release does not constitute an offer to sell, or a solicitation of an offer to buy, any securities in the United States. Small Pharma’s securities have not been and will not be registered under the United States Securities Act of 1933, as amended (the “U.S. Securities Act”) or any state securities laws and may not be offered or sold within the United States or to U.S. Persons unless registered under the U.S. Securities Act and applicable state securities laws or an exemption from such registration is available.
The TSXV has neither approved nor disapproved the contents of this news release. Neither the TSXV nor its Regulation Services Provider (as that term is defined in the policies of the TSXV) accepts responsibility for the adequacy or accuracy of this release.
#dmt, #dmttf